One of the major challenges in liver cancer treatment is early diagnosis. Unfortunately, the symptoms of liver cancer usually appear in the later progression of the disease. As such, most liver cancer cases are diagnosed at an advanced stage, thereby limiting treatment options and lowering overall survival rates. In fact, only one in every five people diagnosed with liver cancer live for at least a year after being diagnosed, and one in every 20 people diagnosed live at least a five years; although there are always exceptions for prolonged life. These alarming numbers stress the importance of regular screening, particularly among those with hepatitis and other risk factors.
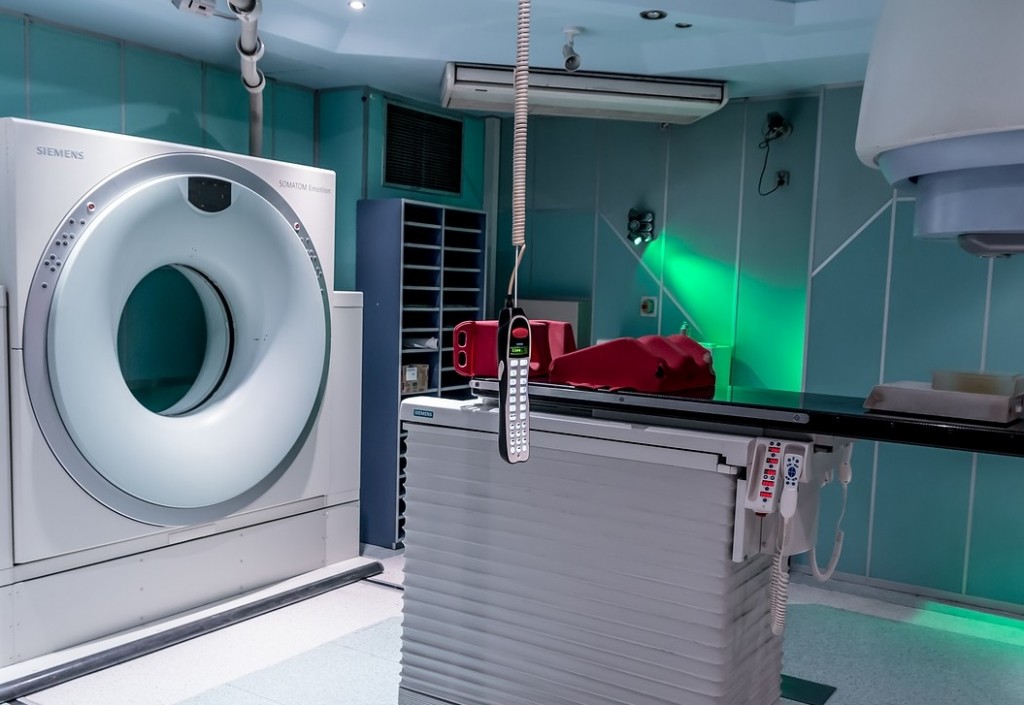
Researchers of a multi-center study have identified a new biomarker that may be used to detect liver metastases at its early stage. This study, published in Science Advances, could have an important and extensive impact, particularly on early and effective diagnosis, and the development of more precise and less risky treatment for liver cancer patients.
“This is a game changer. It has the possibility to have many more applications, really for any type of cancer,” said study corresponding co-author Jenny Yang, a professor at Georgia State University and associate director of the Center for Diagnostics and Therapeutics, in a press release. “We are already applying it to 10 different types of cancer in the lab.”
Using MRI technology, the researchers have developed a protein-based MRI contrast agent that can target certain receptors—specifically the chemokine receptor 4 (CXCR4), which is typically overexpressed in people with metastatic cancer. This CXCR4-targeted agent is expected to permeate existing major barriers and show even small cancer cells on multi-color scans called precision MRI (pMRI), a new imaging methodology.
“Currently, it is difficult to see early stages of disease in the liver, even in invasive biopsy,” Yang explained. “Diagnostic testing using this contrast agent can not only identify the presence of disease but differentiate the stages of disease with high sensitivity and accuracy. That’s the beauty of this work.”
Liver cancer cannot be diagnosed through routine blood tests. A specific blood test for this condition looks at the blood’s alpha-fetoprotein levels, which may be produced by cancer cells. Imaging tests like an ultrasound, CT scan, and MRI scan can be very helpful in looking at liver tumors—but again, on their later stages.
With the promising results of this study, the U.S. Food and Drug Administration is fast-tracking the timeline for clinic trials using the said targeted protein contrast agent to evaluate its efficiency on humans. “We have already met with the FDA, so we have a blueprint,” Yang shared. “We hope within 18 months to two years we can conduct our first clinical trials in patients.”
So, while we wait for updates on this exciting progress in liver cancer research, stay on the side of caution: Screen. Vaccinate. Don’t hesitate!
To learn more about liver cancer treatment and diagnosis, visit our blog
To know more about this research, click here